Foreign manufacturers who are willing to enter the Peruvian medical device market might face the challenge of managing local regulatory procedures. Understanding the Peruvian regulatory framework is essential for successful registration of your medical devices.
The medical device market in Peru is regulated by Dirección General de Medicamentos, Insumos y Drogas (DIGEMID), which is a part of the Ministry of Health (Ministerio de Salud or MINSA).
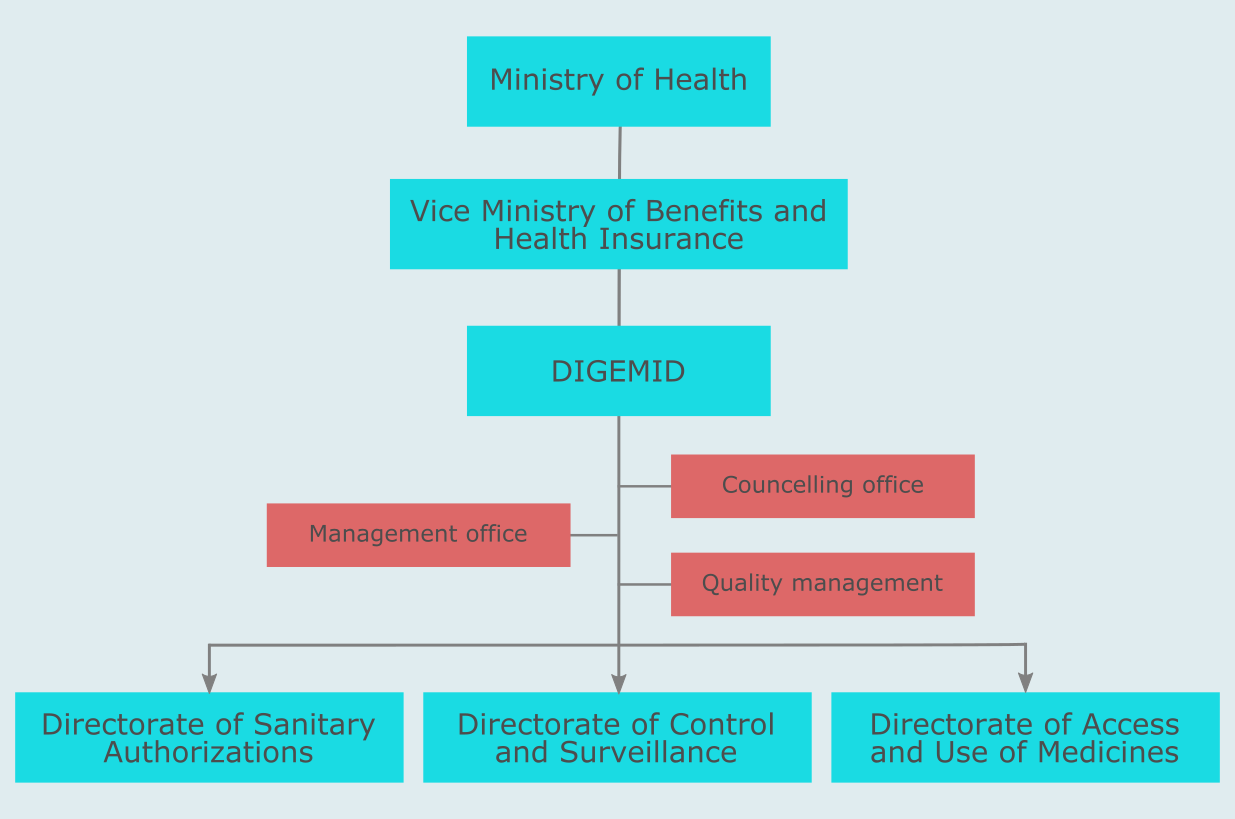
It is the Directorate of Sanitary Authorizations that makes decisions regarding registrations and denials of medical devices in Peru. In general, the Directorate of Sanitary Authorizations is responsible for:
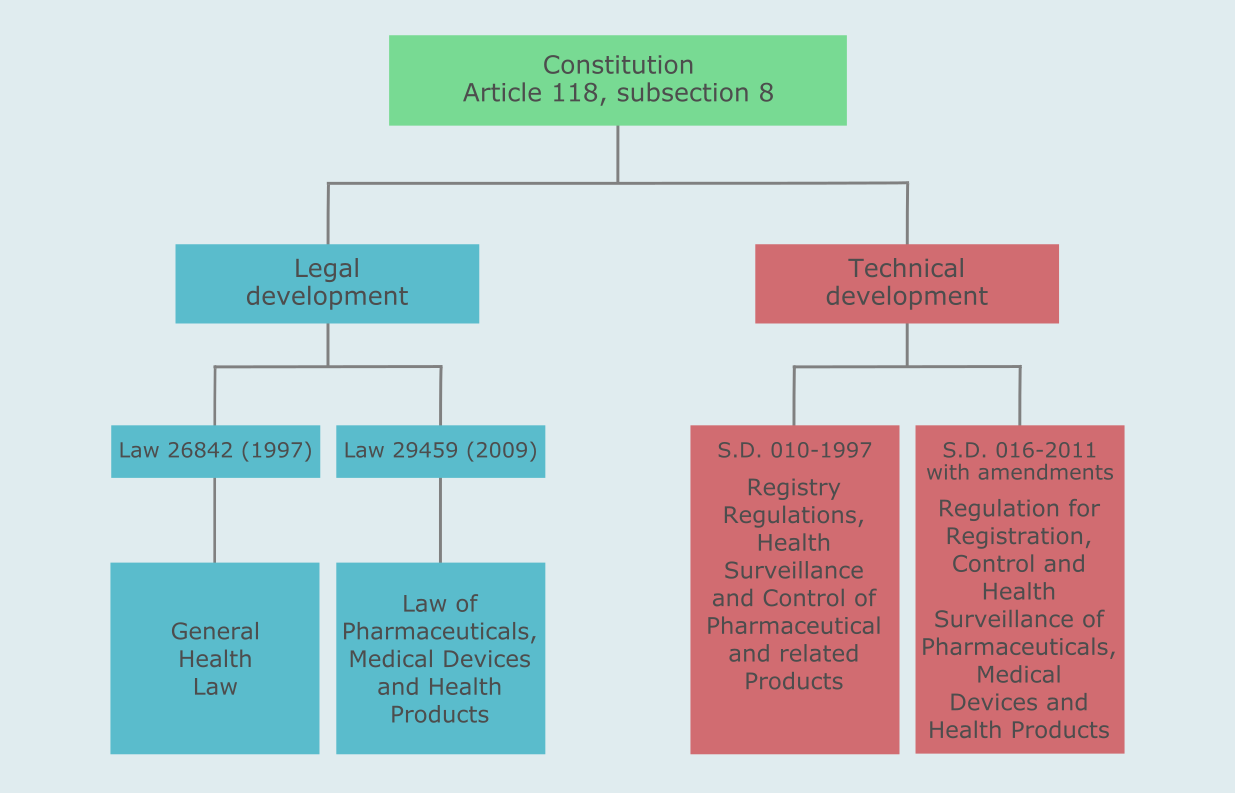
The Peruvian regulatory process for medical devices is based on the Law No. 29459 (Law of Pharmaceuticals, Medical Devices and Health Products) and the Supreme Decree Nº 016-2013-SA (Regulation for Registration, Control and Health Surveillance of Pharmaceutical Products, Medical Devices and Health Products ) with amendments. A number of other regulations are applicable to medical devices in Peru.
Any type of instrument, equipment, implement, reactive, calibrator in vitro or software that has been manufactured to be used in human beings. Those instruments could be used for one or more objectives such as:
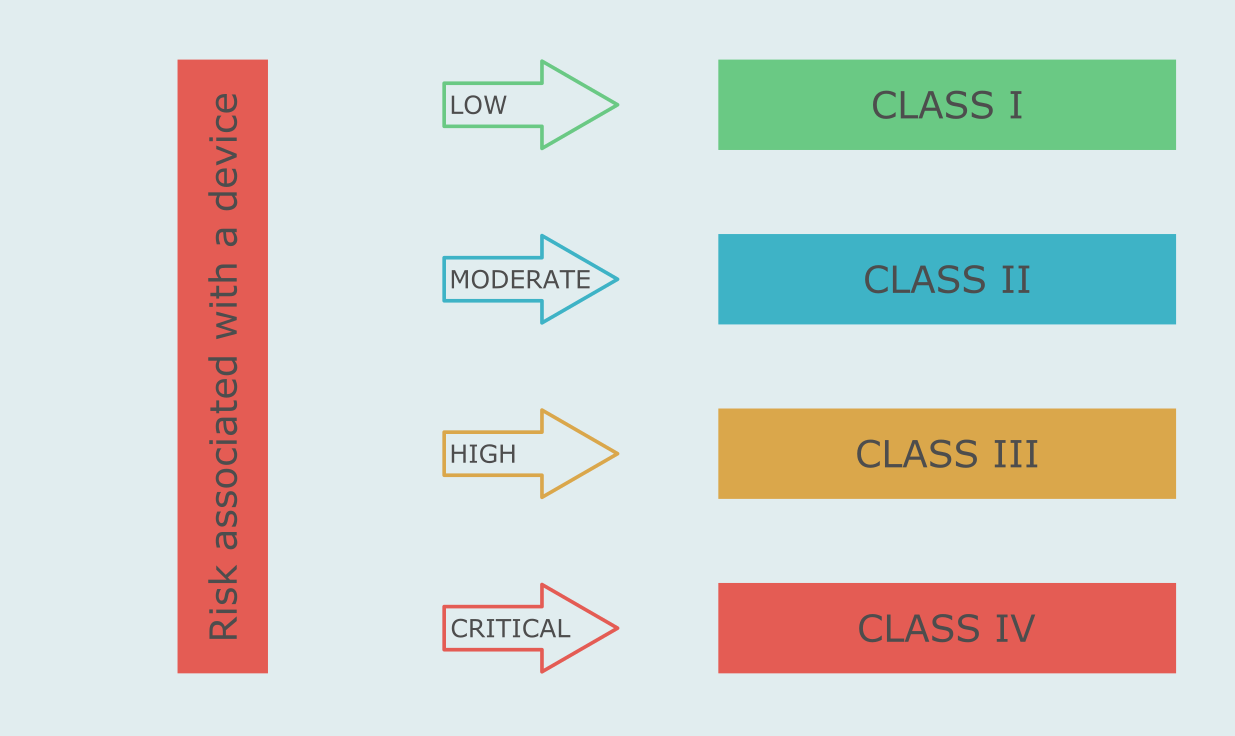
Peruvian classification of medical devices takes into consideration the risks of duration of contact with the body, invasiveness, as well as local and systemic effects of a device. It is based on IMDRF guidelines (GHTF standard).
Under the medical device provisions of the Law 29459 for the purpose of registration manufacturers must provide the following information:
The content and volume of the registration dossier depends on the risk class of a device as shown in the table.
| Requirements | Class I | Class II | Class III | Class IV |
|---|---|---|---|---|
| Application | ✔ | ✔ | ✔ | ✔ |
| CFS/CFG or analogue | ✔ | ✔ | ✔ | ✔ |
| QMS certificate | ✔ | ✔ | ✔ | ✔ |
| Technical report | ✔ | ✔ | ✔ | ✔ |
| Instruction for use | ✔ | ✔ | ✔ | ✔ |
| Quality and security standards (FDA/CE) | ✔ | ✔ | ✔ | |
| Drafts of labels | ✔ | ✔ | ✔ | ✔ |
| Post-marketing surveillance program | ✔ | ✔ | ||
| Risk analysis | ✔ | ✔ | ✔ | |
| List of countries where the device is marketed | ✔ | |||
| Clinical evaluation | ✔ | ✔ | ||
| Technical safety and efficacy information | ✔ | ✔ | ✔ | |
| Biological safety | ✔ | ✔ |
Official medical device registration timelines for medical devices in Peru are:
DIGEMID regitration fees:
DIGEMED has implememnted the VUCE (Single Window of Foreign Trade) electronic system. It facilitetes the coomunication between the regulator and manufacturers of medical devices and can be used for inquiry, consultation, advice and submissions.
The VUCE system is an integrated electronic system that allows parties involved in trade and international transport to manage the documentation required through electronic way. It means, a lot of formalities can be gone through using electronic communication with the Peruvian regulator.
We are here to help you place your medical devices on your strategic markets.
+49 176 67510274
info@mdrc-consulting.com
Foreign manufacturers who are willing to enter the Peruvian medical device market might face the challenge of managing local regulatory procedures. Understanding the Peruvian regulatory framework is essential for successful registration of your medical devices.
The medical device market in Peru is regulated by Dirección General de Medicamentos, Insumos y Drogas (DIGEMID), which is a part of the Ministry of Health (Ministerio de Salud or MINSA).

It is the Directorate of Sanitary Authorizations that makes decisions regarding registrations and denials of medical devices in Peru. In general, the Directorate of Sanitary Authorizations is responsible for:
The Peruvian regulatory process for medical devices is based on the Law No. 29459 (Law of Pharmaceuticals, Medical Devices and Health Products) and the Supreme Decree Nº 016-2013-SA (Regulation for Registration, Control and Health Surveillance of Pharmaceutical Products, Medical Devices and Health Products ) with amendments. A number of other regulations are applicable to medical devices in Peru.

Any type of instrument, equipment, implement, reactive, calibrator in vitro or software that has been manufactured to be used in human beings. Those instruments could be used for one or more objectives such as:
Peruvian classification of medical devices takes into consideration the risks of duration of contact with the body, invasiveness, as well as local and systemic effects of a device. It is based on IMDRF guidelines (GHTF standard).

Under the medical device provisions of the Law 29459 for the purpose of registration manufacturers must provide the following information:
The content and volume of the registration dossier depends on the risk class of a device as shown in the table.
Official medical device registration timelines for medical devices in Peru are:
DIGEMID regitration fees:
DIGEMED has implememnted the VUCE (Single Window of Foreign Trade) electronic system. It facilitetes the coomunication between the regulator and manufacturers of medical devices and can be used for inquiry, consultation, advice and submissions.
The VUCE system is an integrated electronic system that allows parties involved in trade and international transport to manage the documentation required through electronic way. It means, a lot of formalities can be gone through using electronic communication with the Peruvian regulator.
We are here to help you place your medical devices on your strategic markets.
+49 176 67510274
info@mdrc-consulting.com