All medical devices placed on the Russian market must be registered and compliant with local laws and regulations. Foreign manufacturers who are trying to enter the Russian medical device market might face the challenge of managing local regulatory procedures. Understanding the regulatory framework is essential for successful registration of your medical devices in Russia.
The regulatory body responsible for medical devices in Russia is called Roszdravnadzor. It is subordinate to the Ministry of Health of the Russian Federation. And three scientific expert institutions (so called FGBUs - Federal State Institutions) are subordinate to Roszdravnadzor. Medical device registration is the function of the Medical Device Registration and Control Department of this agency.
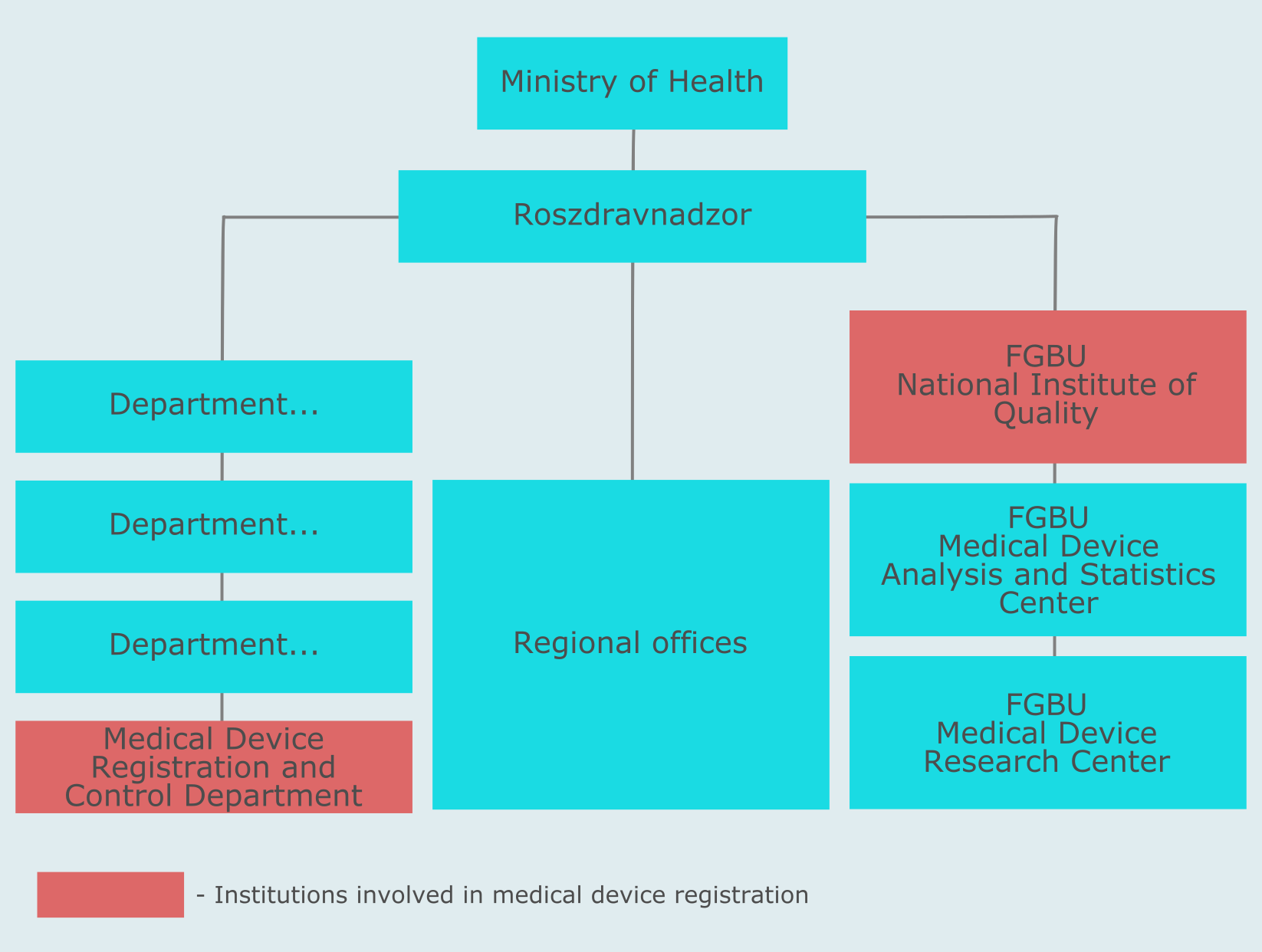
The Medical Device Registration and Control Department makes decisions regarding registrations and denials of medical devices in Russia. It is also responsible for changes and modification approvals, suspension and cancellation of registrations. The document that a medical device manufacturer receives if their registration has been approved is the Registration Certificate.
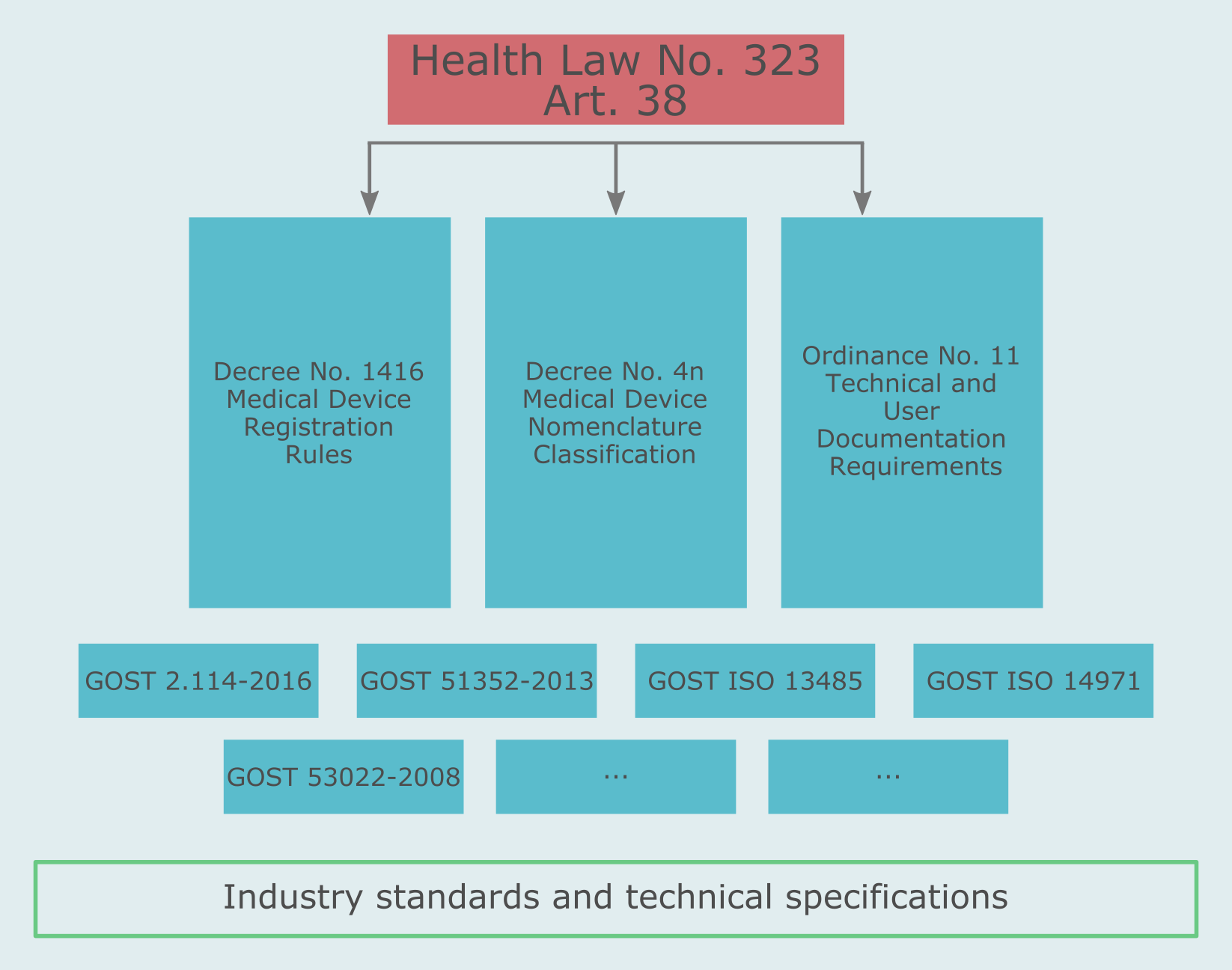
The fundamental provisions for medical devices are set forth in the article 38 of the Health Law (Federal Law 323). Another important regulations are the Decree No. 1416 Medical Device Registration Rules and Decree No. 4n Medical Device Nomenclature Classification, Ministerial Ordinance No. 11 Technical and User Documentation Requirements.
Also, there are a number of product and product family specific standards known as GOSTs. GOST stands for "the state standard". There are national GOSTs called GOST R and GOSTs based on international standards, for instance GOST ISO.
The lowest tier standards used in Russia are industry standards and technical specifications developed by state standardization institutions. Such low tier standards may or may not be applicable to a specific product.
The medical device definition in Russia is very similar to the definition used in the European Union. Under Article 38(1) the term "medical device" is defined as any instrument, apparatus, appliance, material or other article, including the software, intended by its manufacturer to be used for diagnostic and therapeutic purposes (diagnosis, prevention, monitoring, treatment, alleviation or compensation of a disease, injury or handicap), which does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means.
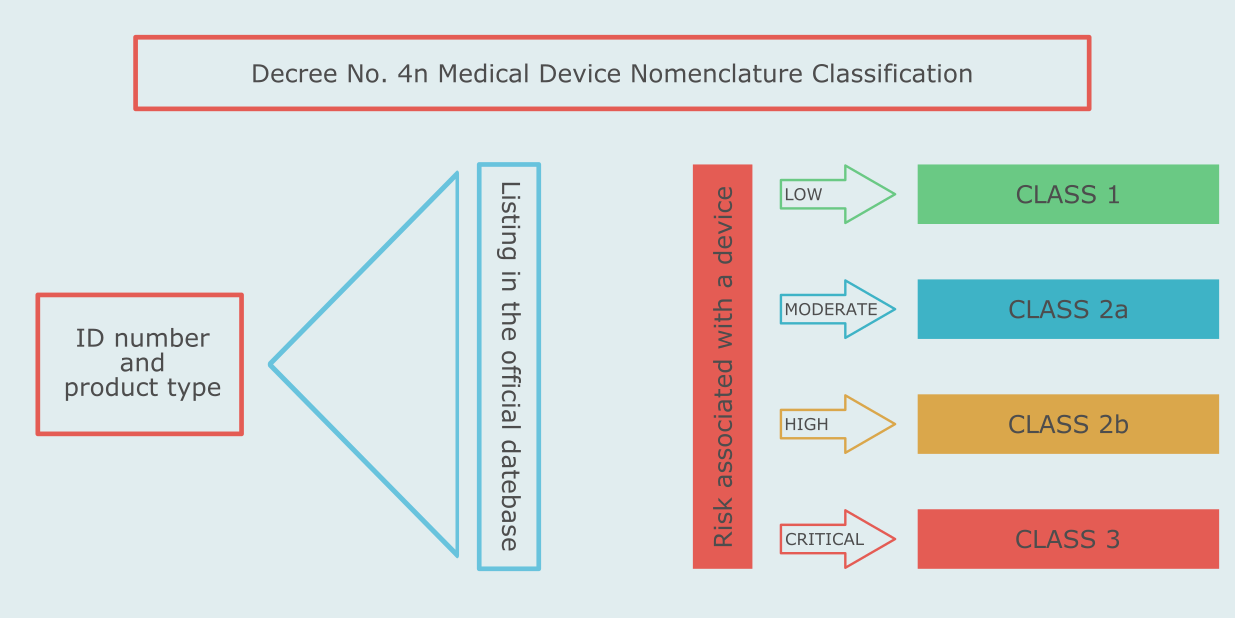
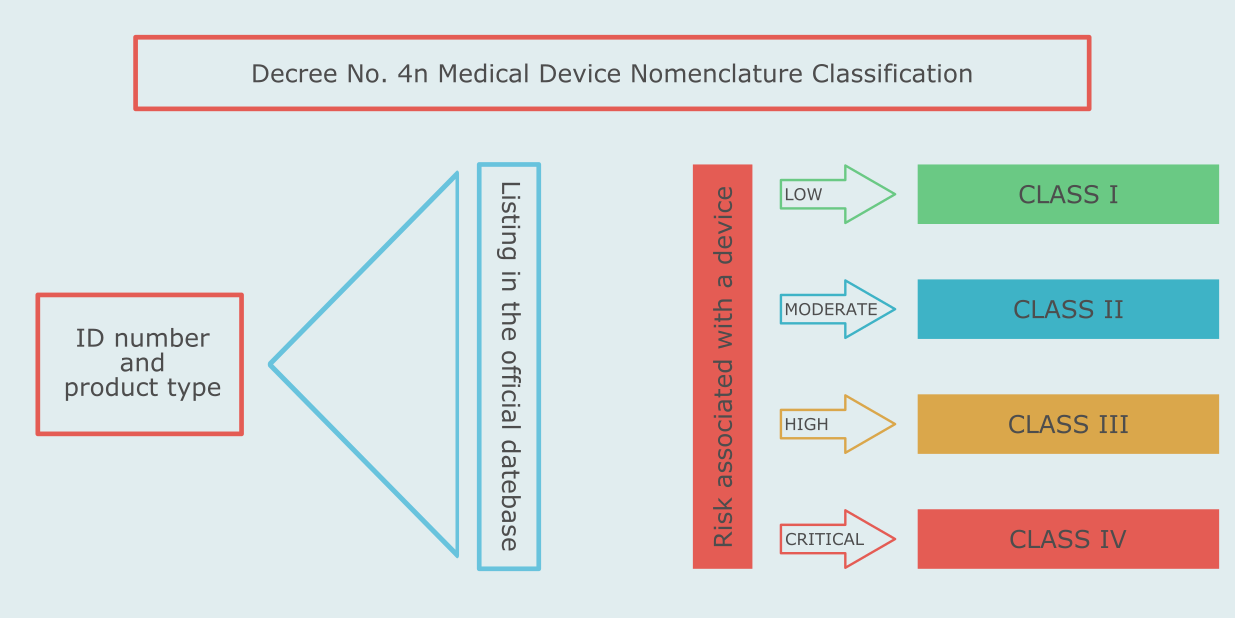
In Russia medical devices are classified by type and by risk associated with their use. The type classification is determined by finding the type of the device in a special list of device types. The risk-based classification of medical devices in Russia is rule-based.
The list of device types and the rules for the risk-based classification are set forth in the Medical Device Nomenclature Classification - Ministry of Health Decree No. 4n.
Like in the European Union, there are four risk classes of medical devices in Russia - 1, 2a, 2b, 3.
For all product classes the applicant should submit to Roszdravnadzor a registration dossier compliant with provisions of the standard Technical and User Documentation Requirements (Ministerial Ordinance No. 11) including:
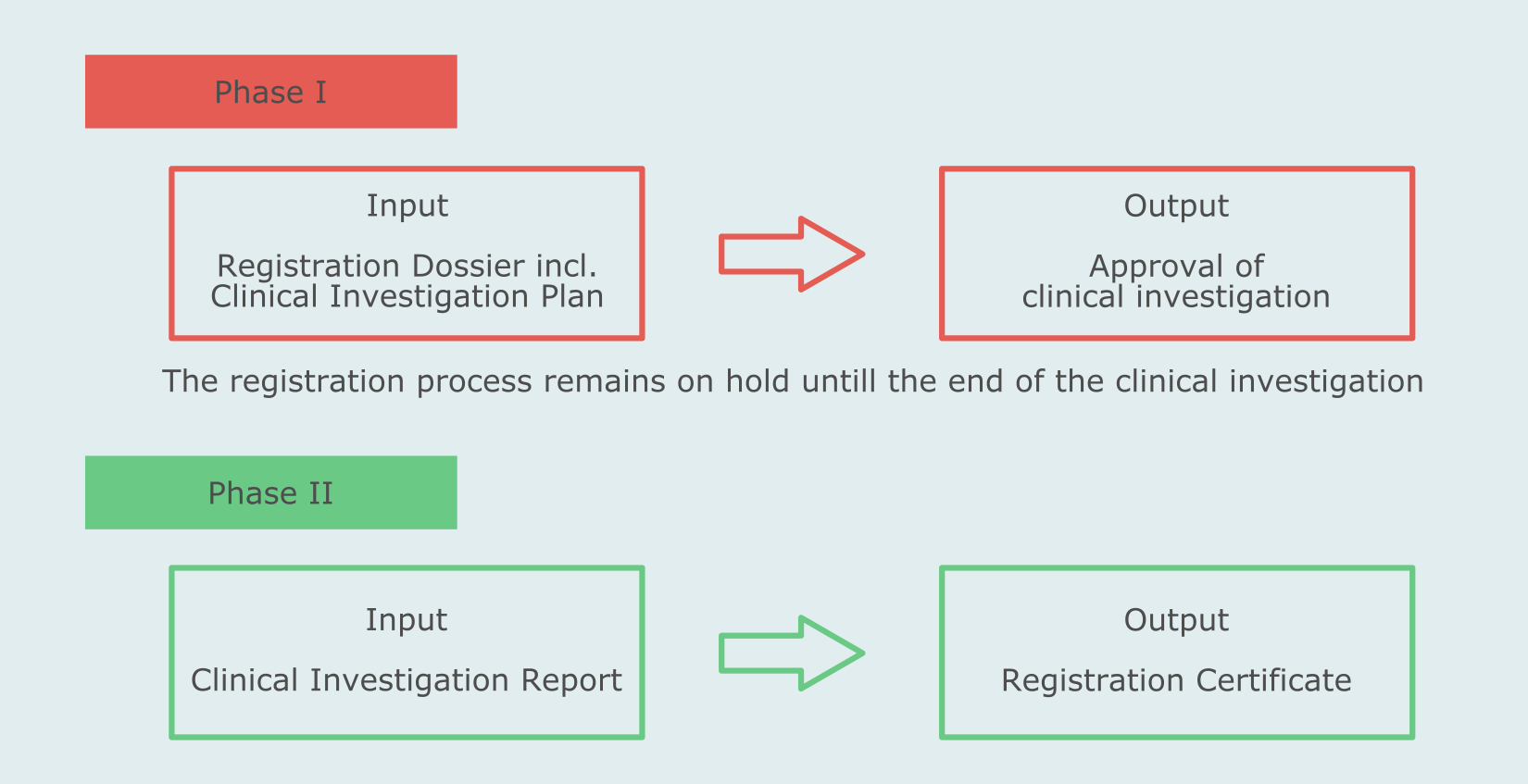
The regulatory approval includes two phases.
We are here to help you place your medical devices on your strategic markets.
+49 176 67510274
info@mdrc-consulting.com
All medical devices placed on the Russian market must be registered and compliant with local laws and regulations. Foreign manufacturers who are trying to enter the Russian medical device market might face the challenge of managing local regulatory procedures. Understanding the regulatory framework is essential for successful registration of your medical devices in Russia.
The regulatory body responsible for medical devices in Russia is called Roszdravnadzor. It is subordinate to the Ministry of Health of the Russian Federation. And three scientific expert institutions (so called FGBUs - Federal State Institutions) are subordinate to Roszdravnadzor. Medical device registration is the function of the Medical Device Registration and Control Department of this agency.

The Medical Device Registration and Control Department makes decisions regarding registrations and denials of medical devices in Russia. It is also responsible for changes and modification approvals, suspension and cancellation of registrations. The document that a medical device manufacturer receives if their registration has been approved is the Registration Certificate.
The fundamental provisions for medical devices are set forth in the article 38 of the Health Law (Federal Law 323). Another important regulations are the Decree No. 1416 Medical Device Registration Rules and Decree No. 4n Medical Device Nomenclature Classification, Ministerial Ordinance No. 11 Technical and User Documentation Requirements.
Also, there are a number of product and product family specific standards known as GOSTs. GOST stands for "the state standard". There are national GOSTs called GOST R and GOSTs based on international standards, for instance GOST ISO.
The lowest tier standards used in Russia are industry standards and technical specifications developed by state standardization institutions. Such low tier standards may or may not be applicable to a specific product.

The medical device definition in Russia is very similar to the definition used in the European Union. Under Article 38(1) the term "medical device" is defined as any instrument, apparatus, appliance, material or other article, including the software, intended by its manufacturer to be used for diagnostic and therapeutic purposes (diagnosis, prevention, monitoring, treatment, alleviation or compensation of a disease, injury or handicap), which does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means.
In Russia medical devices are classified by type and by risk associated with their use. The type classification is determined by finding the type of the device in a special list of device types. The risk-based classification of medical devices in Russia is rule-based.
The list of device types and the rules for the risk-based classification are set forth in the Medical Device Nomenclature Classification - Ministry of Health Decree No. 4n.
Like in the European Union, there are four risk classes of medical devices in Russia - I, IIa, IIb, III.
For all product classes the applicant should submit to Roszdravnadzor a registration dossier compliant with provisions of the standard Technical and User Documentation Requirements (Ministerial Ordinance No. 11) including:
The regulatory approval includes two phases.

We are here to help you place your medical devices on your strategic markets.
+49 176 67510274
info@mdrc-consulting.com