Medical devices in Ecuador are regulated by the national regulatory and surveillance agency and the legal framework comprised of a number of official regulations. All medical devices placed on the Ecuador's market must be registered and compliant with local laws and regulations.
Agencia Nacional de Regulación, Control y Vigilancia Sanitaria (ARCSA) is responsible for medical devices in Ecuador. Accordingly, the medical device registration in Ecuador is regulated by this agency.
There are a number of laws and regulations applicable to medical devices.
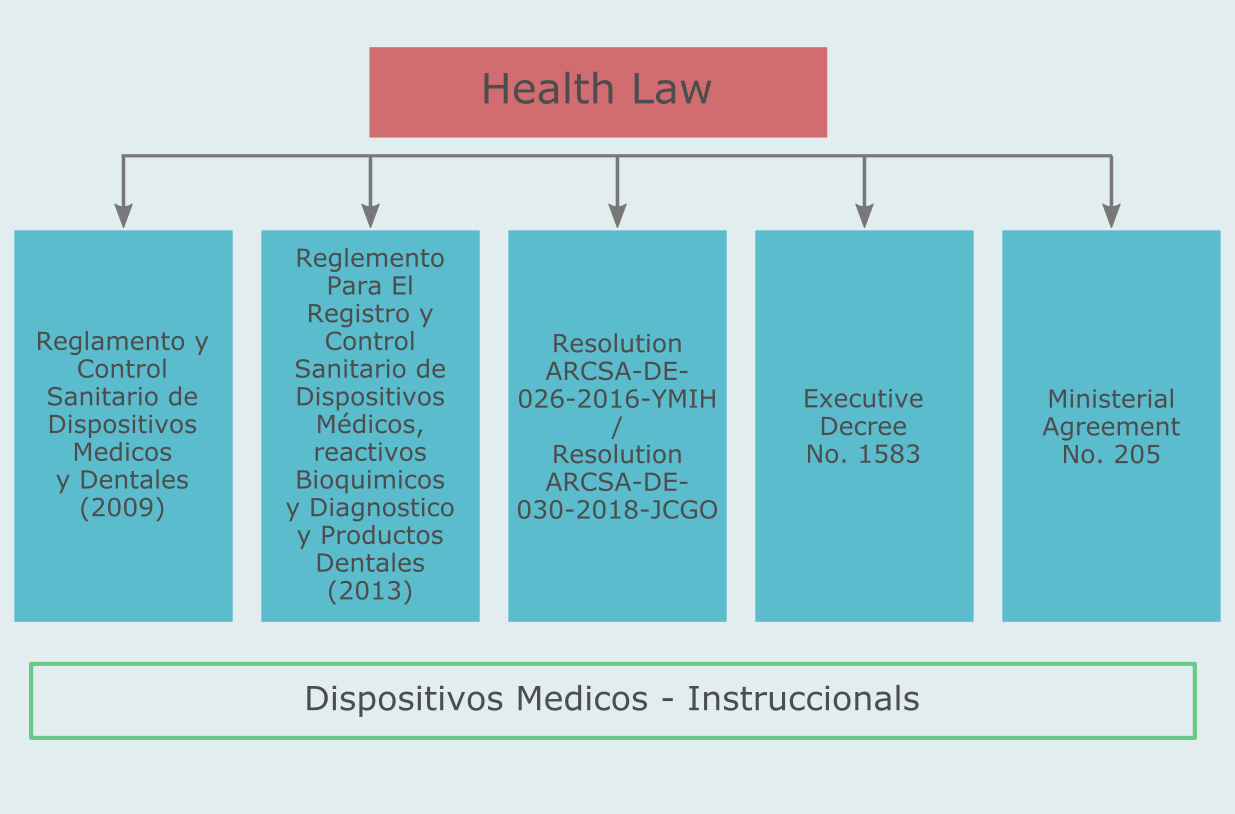
According to Reglemento Para El Registro y Control Sanitario de Dispositivos Médicos, reactivos Bioquimicos y Diagnostico y Productos Dentales (Art. 2): medical devices are articles, instruments, apparatus, appliances and mechanical inventions, including their components, parts or accessories, manufactured, sold or recommended for use in diagnosis, cure or palliative, preventing a disease, disorder or abnormal physical state or its symptoms to replace or modify the anatomy or a physiological process or control. It includes amalgams, varnishes, sealants and similar products and are:
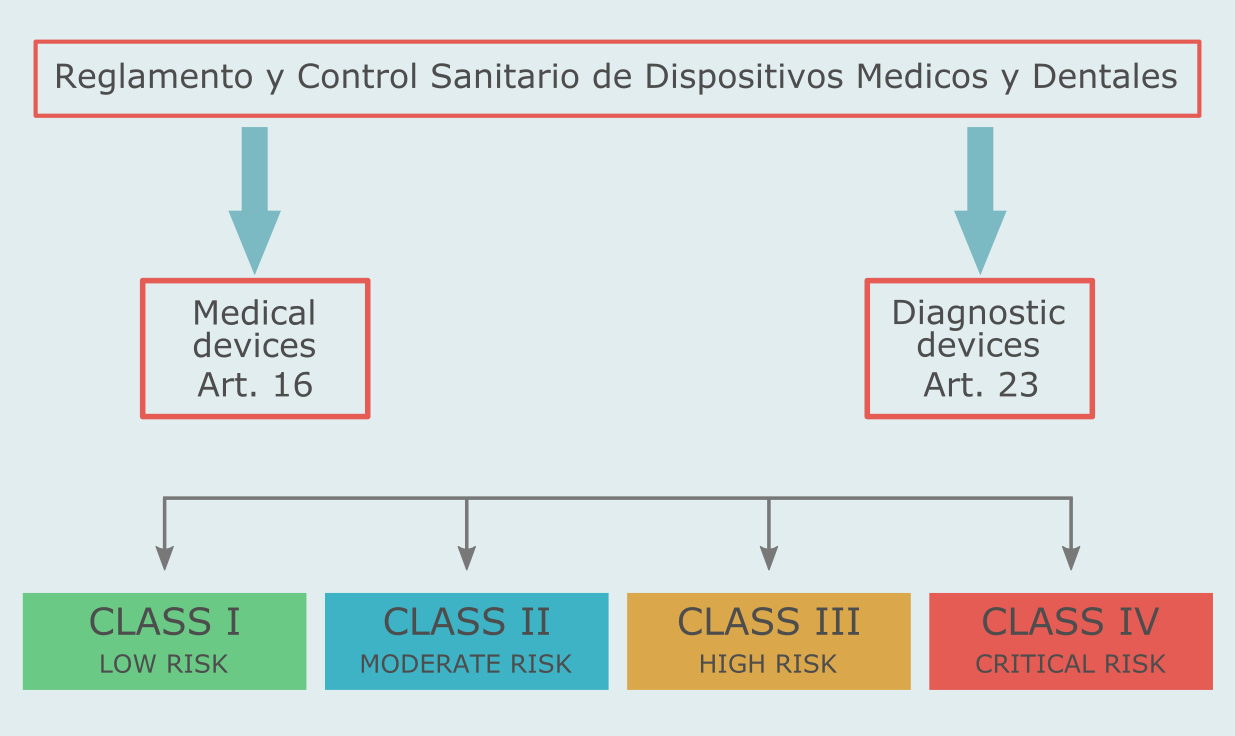
Ecuador's medical device classification is risk- and rule-based. The classification rules are laid down in Article 16 of Reglamento y Control Sanitario de Dispositivos Medicos y Dentales (2009). There are four classes of medical devices in Ecuador: I, II, III, IV.
Diagnostic products are divided into the same four classes, but the classification rules are defined separately - Article 23 of Reglamento y Control Sanitario de Dispositivos Medicos y Dentales (2009).
The following information must be submitted to ARCSA for the purpose of medical device registration.
The standard fee for medical device registration in Ecuador amounts to 904.34 US dollars.
We are here to help you place your medical devices on your strategic markets.
+49 176 67510274
info@mdrc-consulting.com
Medical devices in Ecuador are regulated by the national regulatory and surveillance agency and the legal framework comprised of a number of official regulations. All medical devices placed on the Ecuador's market must be registered and compliant with local laws and regulations.
Agencia Nacional de Regulación, Control y Vigilancia Sanitaria (ARCSA) is responsible for medical devices in Ecuador. Accordingly, the medical device registration in Ecuador is regulated by this agency.
There are a number of laws and regulations applicable to medical devices.

According to Reglemento Para El Registro y Control Sanitario de Dispositivos Médicos, reactivos Bioquimicos y Diagnostico y Productos Dentales (Art. 2): medical devices are articles, instruments, apparatus, appliances and mechanical inventions, including their components, parts or accessories, manufactured, sold or recommended for use in diagnosis, cure or palliative, preventing a disease, disorder or abnormal physical state or its symptoms to replace or modify the anatomy or a physiological process or control. It includes amalgams, varnishes, sealants and similar products and are:
Ecuador's medical device classification is risk- and rule-based. The classification rules are laid down in Article 16 of Reglamento y Control Sanitario de Dispositivos Medicos y Dentales (2009). There are four classes of medical devices in Ecuador: I, II, III, IV.
Diagnostic products are divided into the same four classes, but the classification rules are defined separately - Article 23 of Reglamento y Control Sanitario de Dispositivos Medicos y Dentales (2009).

The following information must be submitted to ARCSA for the purpose of medical device registration.
The standard fee for medical device registration in Ecuador amounts to 904.34 US dollars.
We are here to help you place your medical devices on your strategic markets.
+49 176 67510274
info@mdrc-consulting.com